
Safety and Efficacy
Safety and Efficacy
Brighten your patients’ mornings with Bonjesta®!

Tablets are not actual size

Four Advantages of Bonjesta®
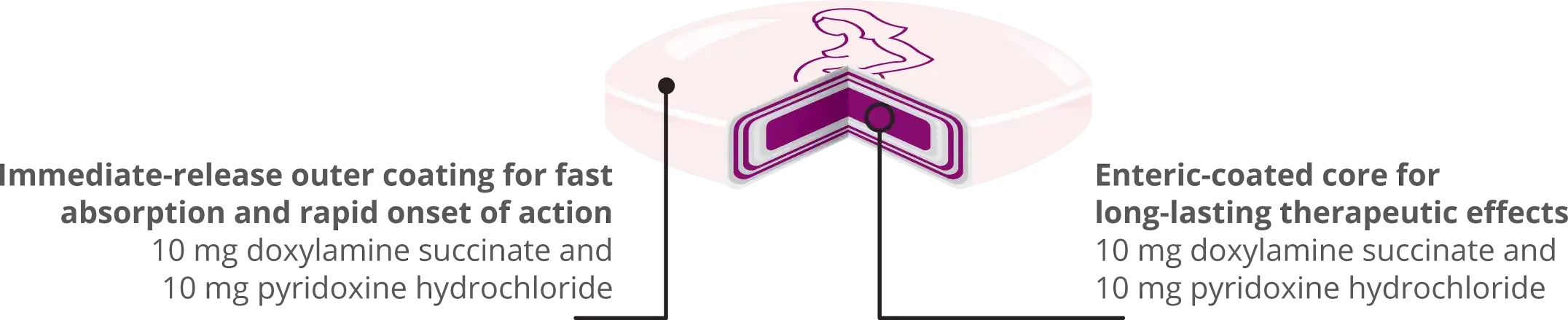
FAST ONSET OF ACTION
Bonjesta® is designed to have a fast onset of action (Tmax) thanks to its immediate release outer coating, providing relief when patients need it most.1,3,5
LONG LASTING RELIEF
Bonjesta® tablets have enteric-coated cores that provide long-lasting therapeutic effects.1,3,5
RECORD OF SAFETY
The combination of ingredients has a 60+ year record of safety for mother and baby and has been used by over 35 million women worldwide.3,4
LESS VARIATION IN EFFECTIVE CONCENTRATIONS
To optimally control NVP, Bonjesta® should be taken daily as prescribed to reduce variations in the effective concentrations of doxylamine and pyridoxal-5-phosphate (PLP) in plasma.1,3
Safety
Two meta-analyses
- 16 cohort and 11 case-controlled studies8
- 12 cohort and 5 case-controlled studies9
Findings
- No statistically significant relationship was found between fetal abnormalities and first trimester use of the combination of doxylamine succinate and pyridoxine hydrochloride ± dicyclomine hydrochloride*1,8
- No differences were found in separate analyses for cardiac defects, limb defects, oral clefts or genital tract malformations1,8

Off-Label Nausea/Vomiting Medications
Other anti-emetics that have been used off-label to manage NVP are not specifically indicated for use throughout pregnancy, primarily because they lack adequate and well-controlled studies in pregnant women and some of them are BOXED WARNING products.
Therefore, off-label medications may NOT be safe for the fetus.
In fact, in 2019, The European Medicines Agency (EMA) updated the summary of product characteristics (SPC) for ondansetron to state that ondansetron should not be used during the first trimester of pregnancy10
Concerns regarding over-the-counter products 7,8

Because doxylamine is present in certain over-the-counter (OTC) products, such as a popular sleep aid, some of your patients may be under the impression that these products can effectively treat NVP.
However, several versions of this OTC brand use different ingredients such as diphenhydramine, acetaminophen and melatonin.
Furthermore, these products:
- Have not been studied, proven safe and effective, or approved by the FDA for use throughout pregnancy
- May contain ingredients that are unsafe for the fetus
- Do not have the critical immediate action combined with extended-release properties
- Are not labeled for use throughout pregnancy and are not indicated for the treatment of NVP when conservative management fails
- Are not available at the correct dosage and require the tablets to be manipulated (split) by the patient
Marijuana Use During Pregnancy

With the growing legalization of marijuana throughout the United States, pregnant women may incorrectly believe that using cannabis to treat their nausea and vomiting of pregnancy symptoms is safe11:
- In Colorado and California, 19-22% of pregnant patients tested positive for cannabis at delivery.
- In pregnant women, cannabis is the most commonly used federally illicit substance
- There is little information available about how fetal cannabis exposure affects developing offspring, however, studies highlighting the risks of fetal exposure to cannabis are increasing.12-15 In human retrospective studies, children exposed to cannabis in-utero were more likely to be admitted to neonatal intensive care units, have lower birth weights, and increased preterm delivery.16
- Preclinical and clinical data show that marijuana use in pregnancy may have harmful effects on the developing fetus.11
Discuss any potential marijuana use with your pregnant patients. Counsel your patients to find alternative treatments for their nausea and vomiting of pregnancy symptoms such as Bonjesta® when conservative management fails to resolve NVP symptoms.
Bonjesta® has not been studied in women with hyperemesis gravidarum.
Efficacy1
Tablet is not actual size
- Bonjesta® offers rapid relief of NVP symptoms and a sustained therapeutic effect, controlling nausea and vomiting symptoms that occur in the morning, during the day, and even into the night.5
- With Bonjesta®, the plasma levels reach therapeutic effect within 1 hour after dosing, conferring a fast-acting relief of symptoms, especially compared to other prescription NVP treatment options that do not reach therapeutic effect until ~6 hours after dosing.5
- Additionally, the dosing frequency for Bonjesta® is at most two pills a day (morning and evening), improving adherence among women during the challenging days of NVP and making it more convenient and easier to follow than other prescription NVP treatment options.6
- Studies in pregnant women have shown that the combination of ingredients in Bonjesta® effectively manages NVP when conservative management fails*. Bonjesta® has not been studied in women with hyperemesis gravidarum.
- The most common adverse reaction is somnolence. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using Bonjesta® until cleared to do so by their healthcare provider.
- There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use. Women should be informed that use of Bonjesta® may result in false positive urine drug screening for methadone, opiates, and PCP.
*Based on the Pregnancy-Unique Quantification of Emesis (PUQE) Score, which evaluates the overall severity of morning sickness: hours of nausea, instances of vomiting and retching over a 24-hour period.
Savings programs for patients are also available!
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
BONJESTA® is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
LIMITATIONS OF USE
BONJESTA® has not been studied in women with hyperemesis gravidarum.
IMPORTANT SAFETY INFORMATION
Contraindications:
BONJESTA® is contraindicated in women with any of the following conditions:
- Known hypersensitivity to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any inactive ingredient in the formulation;
- Monoamine oxidase (MAO) inhibitors intensify and prolong the adverse central nervous system effects of BONJESTA®.
Warnings and Precautions:
- Somnolence: BONJESTA® may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA® until cleared to do so by their healthcare provider.
- Central nervous system (CNS) depressants: Use of BONJESTA® is not recommended if a woman is concurrently using CNS depressants, such as alcohol or sedating medications, including other antihistamines (present in some cough and cold medications), opiates, and sleep aids. The combination of BONJESTA® and CNS depressants could result in severe drowsiness leading to falls or other accidents.
- Concomitant Medical Conditions: BONJESTA® has anticholinergic properties and, therefore, should be used with caution in women with increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, or bladder-neck obstruction.
- Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP): There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use. Women should be informed that use of BONJESTA® may result in false positive urine drug screening for methadone, opiates, and PCP.
Adverse Reactions:
The most common adverse reaction (≥5 percent and exceeding the rate in placebo) is somnolence.
Drug-Food Interactions:
BONJESTA® should be taken on an empty stomach with a glass of water.
Drug Interactions:
Severe drowsiness can occur when used in combination with alcohol or other sedating medications.
Use in Specific Populations:
- Pregnancy: BONJESTA® is intended for use in pregnant women.
- Lactation: Women should not breastfeed while using BONJESTA® because the antihistamine component (doxylamine succinate) in BONJESTA® can pass into breast milk. Excitement, irritability, and sedation have been reported in nursing infants presumably exposed to doxylamine succinate through breast milk. Infants with apnea or other respiratory syndromes may be particularly vulnerable to the sedative effects of BONJESTA®, resulting in worsening of their apnea or respiratory conditions.
- Pediatric Use: The safety and effectiveness of BONJESTA® in children under 18 years of age have not been established. Fatalities have been reported from doxylamine overdose in children. Children appear to be at a high risk for cardiorespiratory arrest.
Overdosage:
BONJESTA® is an extended-release formulation; therefore, signs and symptoms of intoxication may not be apparent immediately. Signs and symptoms of overdose may include restlessness, dryness of mouth, dilated pupils, sleepiness, vertigo, mental confusion, and tachycardia. At toxic doses, doxylamine exhibits anticholinergic effects, including seizures, rhabdomyolysis, acute renal failure, and death. If treatment is needed, it consists of gastric lavage or activated charcoal, whole bowel irrigation, and symptomatic treatment. If you suspect an overdose or seek additional information about overdose treatment, call a poison control center at 1-800-222-1222.
To report suspected adverse reactions, contact Duchesnay Inc. at 1-855-722-7734 or [email protected] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References
- Bonjesta® (doxylamine succinate and pyridoxine hydrochloride), extended-release tablets, for oral use. [Prescribing Information]. Duchesnay USA, Inc.
- ACOG Practice Bulletin No. 189: Nausea and Vomiting of Pregnancy. Clinical Management Guidelines for Obstetrician-Gynecologists. 2018;131(1):e15-30.
- Duchesnay, Inc. Data on File.
- APGO Continuing Series on Nausea and Vomiting of Pregnancy. Rockville, MD. 2014.
- Schleußner, Ekkehard et al. “Nausea and Vomiting of Pregnancy and its Management with the Dual-Release Formulation of Doxylamine and Pyridoxine.” Geburtshilfe und Frauenheilkunde vol. 84,2 144-152. 8 Feb. 2024, doi:10.1055/a-2225-5883
- Constantine, Maged M et al. “Determinants of adherence to delayed-release doxylamine and pyridoxine in patients with nausea and vomiting of pregnancy.” Therapeutic drug monitoring vol. 34,5 (2012): 569-73. doi:10.1097/FTD.0b013e31826e7997
- Madjunkova, S., Maltepe, C., & Koren, G. The Delayed Release Combination of Doxylamine and Pyridoxine (Diclegis®/Diclectin®) for the Treatment of Nausea and Vomiting of Pregnancy. Pediatr Drugs 2014; 16(3): 199-211.
- McKeigue PM, et al. Bendectin and birth defects: I. A meta-analysis of the epidemiologic studies. Teratology 1994;50:27–37.
- Einarson TR, et al. A method for meta-analysis of epidemiological studies. Drug Intell Clin Pharm 1988;22(10):813–24.
- Ondansetron 4 mg film-coated tablets. Summary of Product Characteristics. Updated 30-Aug-2024. Aurobindo Pharma – Milpharm Ltd.
- Swenson, K. Cannabis for morning sickness: areas for intervention to decrease cannabis consumption during pregnancy. J Cannabis Res 5, 22 (2023).
- Chia-Shan Wu, Christopher P Jew and Hui-Chen Lu. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6(4).
- Viveros MP, Marco E. Age-dependent effects of cannabinoids on neurophysiological, emotional, and motivational states. In: Campolongo P., Fattore L. (Eds) Cannabinoid modulation of emotion, memory, and motivation. New York: Springer. 2015.
- Jansson LM, Jordan CJ, Velez ML. Perinatal marijuana use and the developing child. JAMA. 2018;320(6):545–6.
- Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, Melis M. Cannabis and the developing brain: insights into its long-lasting effects. Journal of Neuroscience. 2019;39(42):8250–8.
- Marchand G, Masoud AT, Govindan M, Ware K, King A, Ruther S, Brazil G, Ulibarri H, Parise J, Arroyo A, Coriell C, Goetz S, Karrys A, Sainz K. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(1):e2145653.
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
BONJESTA® is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
LIMITATIONS OF USE
BONJESTA® has not been studied in women with hyperemesis gravidarum.