
Nausea And Vomiting of Pregnancy (NVP)
Nausea And Vomiting of Pregnancy (NVP)
About NVP
NVP is the most common medical condition associated with pregnancy, affecting 50-85% of all pregnant women 2-4,6.
About 60% of women suffering from NVP rated the severity of symptoms as moderate or severe 5,14.
Additionally, NVP can result in adverse effects 6.
Physical consequences
Emotional consequences
Financial consequences
Once conservative management has failed to resolve your patient’s NVP symptoms, early pharmacologic treatment is recommended, as treatment delay may lead to increased difficulty in managing symptoms and potentially further complications for your patient 2,8.
Symptoms

NVP is commonly called “morning sickness”, but that term is misleading as it implies that symptoms only occur in the morning, and this may trivialize the condition for many women suffering from it2,9.
It more accurately could be called “episodic daytime pregnancy sickness” due to the episodic pattern of NVP5,6.
Nausea and vomiting symptoms can occur throughout the day. The majority of women present symptoms before and after midday5,9.
While vomiting mostly occurs in the mornings, nausea, which many sufferers describe as the worst aspect of their symptoms, can occur at any time of the day9.
A prospective study of 160 women reported that the severity of nausea was comparable to that induced by chemotherapy11.
NVP is commonly called “morning sickness”, but that term is misleading as it implies that symptoms only occur in the morning, and this may trivialize the condition for many women suffering from it2,9.
It more accurately could be called “episodic daytime pregnancy sickness” due to the episodic pattern of NVP5,6.
Nausea and vomiting symptoms can occur throughout the day. The majority of women present symptoms before and after midday5,9.


While vomiting mostly occurs in the mornings, nausea, which many sufferers describe as the worst aspect of their symptoms, can occur at any time of the day.9
A prospective study of 160 women reported that the severity of nausea was comparable to that induced by chemotherapy.11
Symptoms of NVP may include:
Nausea
Vomiting
Retching
These symptoms can vary from mild to severe, with about 60% of women suffering from NVP rating the severity of symptoms as moderate or severe.5,14
During pregnancy, all women should be screened for NVP, and its severity can be assessed using the validated Pregnancy Unique Quantification of Emesis (PUQE) test.1,7
Included in most NVP guidelines, this test is based on the number of episodes of nausea, vomiting and retching the patient experiences over a 24-hour period.1,7

Ask your patient the questions and answers shown below to assess the severity of your patient’s NVP symptoms.
In the last 24 hours, how long has your patient felt nauseous or sick to their stomach?
In the last 24 hours, has your patient vomited or thrown up?
In the last 24 hours, how many times has your patient had retching or dry heaves without bringing anything up?
Clinical Course7,8
Percentage of patients with nausea and vomiting by gestational week8
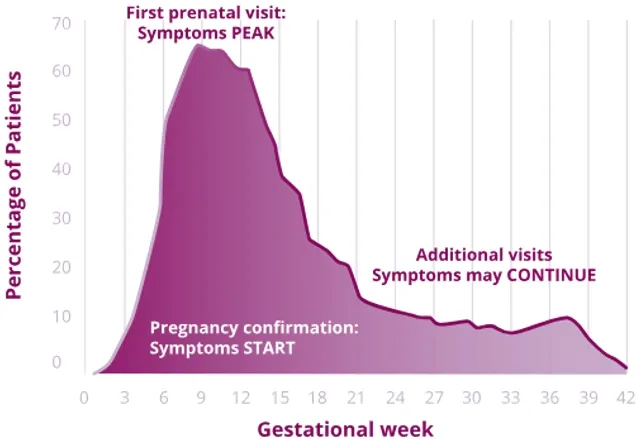
NVP typically appears before week 9 and for many women, NVP resolves around weeks 16-22 of gestation.7,11
However, for about 10-20% of women, NVP can last throughout the entire pregnancy.7
The most severe form of NVP, hyperemesis gravidarum (HG) occurs in 0.3-3% of women.*7
Impact of NVP
PHYSICAL CONSEQUENCES
- 30-40% of pregnant women are not able to fully participate in family and social activities due to NVP6
- 621 pregnant women surveyed revealed that NVP has strongly affected their eating behavior, pleasure, diet, and sleep6,15
- NVP may influence the willingness of women to conceive again6
- The severity of nausea experienced during NVP was comparable to that experienced during chemotherapy for 160 women in a prospective study11
EMOTIONAL CONSEQUENCES
- Women experiencing NVP have elevated anxiety levels with more prominent symptoms seen in women experiencing moderate to severe NVP symptoms3
- Adverse psychological factors are associated with HG* or NVP such as5:
- Depression
- Anxiety
- Mood disorders
- Stress
FINANCIAL & ECONOMIC CONSEQUENCES
- Economic impact through time lost in paid work by women suffering from NVP:
- In one United Kingdom study, around 35% of females in paid work lost time from the condition, causing an estimate loss of 8.6 million hours of paid work per year9.
- In 2012 the United States spent nearly $2 billion in costs attributed to pregnancy-related nausea and vomiting10,16:
- 60% of this expenditure is a result of direct costs (e.g., drugs, hospital admission)
- 40% is a result of indirect costs (e.g., time lost from work).
Pathophysiology
In recent years, evidence has suggested that NVP and HG* are caused by high levels of Growth-Differentiation Factor 15 (GDF15), a hormone secreted by the placenta starting in early pregnancy5,12, which is beneficial to fetal survival and growth.
Higher levels of GDF15 are causally associated with stronger NVP.12
Other factors that may contribute to NVP are12,13:
- IGFBP7 (Insulin-like growth factor-binding protein 7)
- other hormones
- PGR (progesterone receptor)
- helicobacter pylori
- Human choronic gonadotropin (hCG)
- gastrointestinal dysmotility
- placenta related factors
- thyroid hormone
- psychosocial factors.
Conservative Management2
The treatment of NVP begins with prevention. Taking prenatal vitamins for 1 month before pregnancy may reduce the incidence and severity of NVP for your patient.
Once NVP symptoms have appeared, some diet and lifestyle modifications are recommended:
- Frequent, small meals every 1-2 hours to avoid a full stomach
- Avoiding spicy or fatty foods
- Eliminating supplemental iron and substituting folic acid for iron-containing prenatal vitamins
- Eating bland or dry foods, high-protein snacks, and crackers in the morning before arising.
- Getting adequate rest
- Avoidance of sensory stimuli such as odors, heat, humidity, noise, and flickering lights that may provoke symptoms
- Ginger has shown some beneficial effects in reducing nausea symptoms
- Acupressure, acupuncture, or electrical nerve stimulation (acustimulation) at the P6 or Neiguan point
Better mornings with Bonjesta®
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
BONJESTA® is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
LIMITATIONS OF USE
BONJESTA® has not been studied in women with hyperemesis gravidarum.
IMPORTANT SAFETY INFORMATION
Contraindications:
BONJESTA® is contraindicated in women with any of the following conditions:
- Known hypersensitivity to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any inactive ingredient in the formulation;
- Monoamine oxidase (MAO) inhibitors intensify and prolong the adverse central nervous system effects of BONJESTA®.
Warnings and Precautions:
- Somnolence: BONJESTA® may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA® until cleared to do so by their healthcare provider.
- Central nervous system (CNS) depressants: Use of BONJESTA® is not recommended if a woman is concurrently using CNS depressants, such as alcohol or sedating medications, including other antihistamines (present in some cough and cold medications), opiates, and sleep aids. The combination of BONJESTA® and CNS depressants could result in severe drowsiness leading to falls or other accidents.
- Concomitant Medical Conditions: BONJESTA® has anticholinergic properties and, therefore, should be used with caution in women with increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, or bladder-neck obstruction.
- Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP): There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use. Women should be informed that use of BONJESTA® may result in false positive urine drug screening for methadone, opiates, and PCP.
Adverse Reactions:
The most common adverse reaction (≥5 percent and exceeding the rate in placebo) is somnolence.
Drug-Food Interactions:
BONJESTA® should be taken on an empty stomach with a glass of water.
Drug Interactions:
Severe drowsiness can occur when used in combination with alcohol or other sedating medications.
Use in Specific Populations:
- Pregnancy: BONJESTA® is intended for use in pregnant women.
- Lactation: Women should not breastfeed while using BONJESTA® because the antihistamine component (doxylamine succinate) in BONJESTA® can pass into breast milk. Excitement, irritability, and sedation have been reported in nursing infants presumably exposed to doxylamine succinate through breast milk. Infants with apnea or other respiratory syndromes may be particularly vulnerable to the sedative effects of BONJESTA®, resulting in worsening of their apnea or respiratory conditions.
- Pediatric Use: The safety and effectiveness of BONJESTA® in children under 18 years of age have not been established. Fatalities have been reported from doxylamine overdose in children. Children appear to be at a high risk for cardiorespiratory arrest.
Overdosage:
BONJESTA® is an extended-release formulation; therefore, signs and symptoms of intoxication may not be apparent immediately. Signs and symptoms of overdose may include restlessness, dryness of mouth, dilated pupils, sleepiness, vertigo, mental confusion, and tachycardia. At toxic doses, doxylamine exhibits anticholinergic effects, including seizures, rhabdomyolysis, acute renal failure, and death. If treatment is needed, it consists of gastric lavage or activated charcoal, whole bowel irrigation, and symptomatic treatment. If you suspect an overdose or seek additional information about overdose treatment, call a poison control center at 1-800-222-1222.
To report suspected adverse reactions, contact Duchesnay Inc. at 1-855-722-7734 or [email protected] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References
- Bonjesta® (doxylamine succinate and pyridoxine hydrochloride), extended-release tablets, for oral use. [Prescribing Information]. Duchesnay USA, Inc.
- ACOG Practice Bulletin No. 189: Nausea and Vomiting of Pregnancy. Clinical Management Guidelines for Obstetrician-Gynecologists. 2018;131(1):e15-30.
- Beyazit, Fatma, and Basak Sahin. “Effect of Nausea and Vomiting on Anxiety and Depression Levels in Early Pregnancy.” The Eurasian journal of medicine vol. 50,2 (2018): 111-115. doi:10.5152/eurasianjmed.2018.170320
- Gadsby, Roger et al. “Nausea and vomiting of pregnancy and resource implications: the NVP Impact Study.” The British journal of general practice vol. 69,680 (2019): e217-e223. doi:10.3399/bjgp18X700745
- Liu, Chuan et al. “Emerging Progress in Nausea and Vomiting of Pregnancy and Hyperemesis Gravidarum: Challenges and Opportunities.” Frontiers in medicine vol. 8 809270. 10 Jan. 2022, doi:10.3389/fmed.2021.809270
- Schleußner, Ekkehard et al. “Nausea and Vomiting of Pregnancy and its Management with the Dual-Release Formulation of Doxylamine and Pyridoxine.” Geburtshilfe und Frauenheilkunde vol. 84,2 144-152. 8 Feb. 2024, doi:10.1055/a-2225-5883
- Tinti, Serena et al. “Prevalence and burden of nausea and vomiting in pregnant women: Interim analysis of the PURITY survey.” European journal of obstetrics, gynecology, and reproductive biology vol. 290 (2023): 135-142. doi:10.1016/j.ejogrb.2023.09.016
- Vellacott, I D et al. “Nausea and vomiting in early pregnancy.” International journal of gynaecology and obstetrics vol. 27,1 (1988): 57-62. doi:10.1016/0020-7292(88)90088-4
- Gadsby, Roger et al. “Nausea and vomiting in pregnancy is not just 'morning sickness': data from a prospective cohort study in the UK.” The British journal of general practice vol. 70,697 e534-e539. 30 Jul. 2020, doi:10.3399/bjgp20X710885
- McCarthy, Fergus P et al. “Hyperemesis gravidarum: current perspectives.” International journal of women's health vol. 6 719-25. 5 Aug. 2014, doi:10.2147/IJWH.S37685
- Lacroix, R et al. “Nausea and vomiting during pregnancy: A prospective study of its frequency, intensity, and patterns of change.” American journal of obstetrics and gynecology vol. 182,4 (2000): 931-7. doi:10.1016/S0002-9378(00)70349-8
- Crespi, Bernard J. “Nausea, vomiting and conflict in pregnancy: The adaptive significance of Growth-Differentiation Factor 15.” Evolution, medicine, and public health vol. 12,1 75-81. 23 Apr. 2024, doi:10.1093/emph/eoae008
- Fejzo, Marlena S et al. “Nausea and vomiting of pregnancy and hyperemesis gravidarum.” Nature reviews. Disease primers vol. 5,1 62. 12 Sep. 2019, doi:10.1038/s41572-019-0110-3
- Einarson TR, Piwko C, Koren G. Quantifying the global rates of nausea and vomiting of pregnancy: a meta analysis. J Popul Ther Clin Pharmacol (2013) 20:e171-83.
- Clark S, Hughes B, McDonald SS. The impact of nausea and vomiting of pregnancy on quality of life. Report of a national consumer survey and recommendations for improving care. Obstet Gynecol Surv 2013; 68 (Suppl 1): S1-S10.
- Piwko C, Koren G, Babashov V, Vicente C, Einarson TR. Economic burden of nausea and vomiting of pregnancy in the USA. J Popul Ther Clin Pharmacol. 2013;20(2):e149–e160.
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
BONJESTA® is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
LIMITATIONS OF USE
BONJESTA® has not been studied in women with hyperemesis gravidarum.
