INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
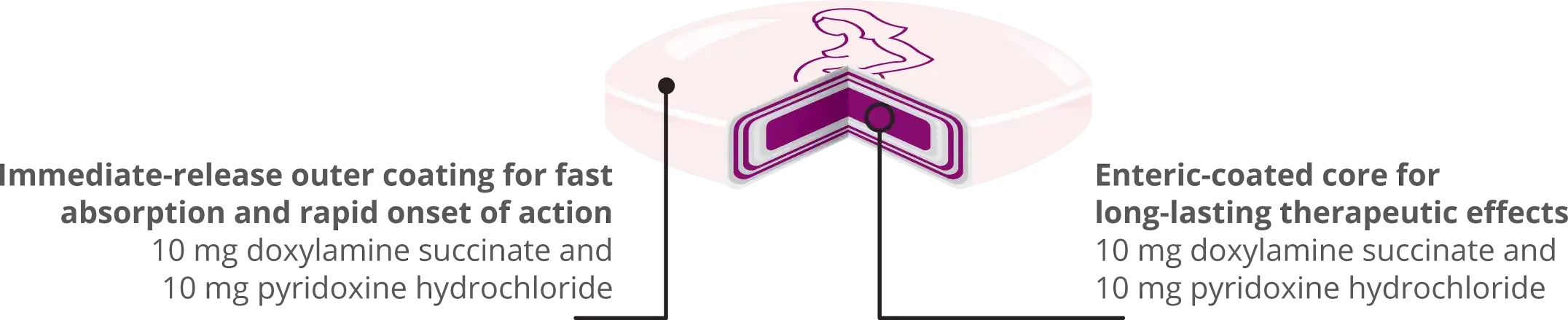
BONJESTA® is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management.
LIMITATIONS OF USE
BONJESTA® has not been studied in women with hyperemesis gravidarum.
IMPORTANT SAFETY INFORMATION
Contraindications:
BONJESTA® is contraindicated in women with any of the following conditions:
- Known hypersensitivity to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any inactive ingredient in the formulation;
- Monoamine oxidase (MAO) inhibitors intensify and prolong the adverse central nervous system effects of BONJESTA®.
Warnings and Precautions:
- Somnolence: BONJESTA® may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA® until cleared to do so by their healthcare provider.
- Central nervous system (CNS) depressants: Use of BONJESTA® is not recommended if a woman is concurrently using CNS depressants, such as alcohol or sedating medications, including other antihistamines (present in some cough and cold medications), opiates, and sleep aids. The combination of BONJESTA® and CNS depressants could result in severe drowsiness leading to falls or other accidents.
- Concomitant Medical Conditions: BONJESTA® has anticholinergic properties and, therefore, should be used with caution in women with increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, or bladder-neck obstruction.
- Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP): There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use. Women should be informed that use of BONJESTA® may result in false positive urine drug screening for methadone, opiates, and PCP.
Adverse Reactions:
The most common adverse reaction (≥5 percent and exceeding the rate in placebo) is somnolence.
Drug-Food Interactions:
BONJESTA® should be taken on an empty stomach with a glass of water.
Drug Interactions:
Severe drowsiness can occur when used in combination with alcohol or other sedating medications.
Use in Specific Populations:
- Pregnancy: BONJESTA® is intended for use in pregnant women.
- Lactation: Women should not breastfeed while using BONJESTA® because the antihistamine component (doxylamine succinate) in BONJESTA® can pass into breast milk. Excitement, irritability, and sedation have been reported in nursing infants presumably exposed to doxylamine succinate through breast milk. Infants with apnea or other respiratory syndromes may be particularly vulnerable to the sedative effects of BONJESTA®, resulting in worsening of their apnea or respiratory conditions.
- Pediatric Use: The safety and effectiveness of BONJESTA® in children under 18 years of age have not been established. Fatalities have been reported from doxylamine overdose in children. Children appear to be at a high risk for cardiorespiratory arrest.
Overdosage:
BONJESTA® is an extended-release formulation; therefore, signs and symptoms of intoxication may not be apparent immediately. Signs and symptoms of overdose may include restlessness, dryness of mouth, dilated pupils, sleepiness, vertigo, mental confusion, and tachycardia. At toxic doses, doxylamine exhibits anticholinergic effects, including seizures, rhabdomyolysis, acute renal failure, and death. If treatment is needed, it consists of gastric lavage or activated charcoal, whole bowel irrigation, and symptomatic treatment. If you suspect an overdose or seek additional information about overdose treatment, call a poison control center at 1-800-222-1222.
To report suspected adverse reactions, contact Duchesnay Inc. at 1-855-722-7734 or [email protected] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.